Transcriptional non-oncogene addiction: A major obstacle to the success of targeted therapies
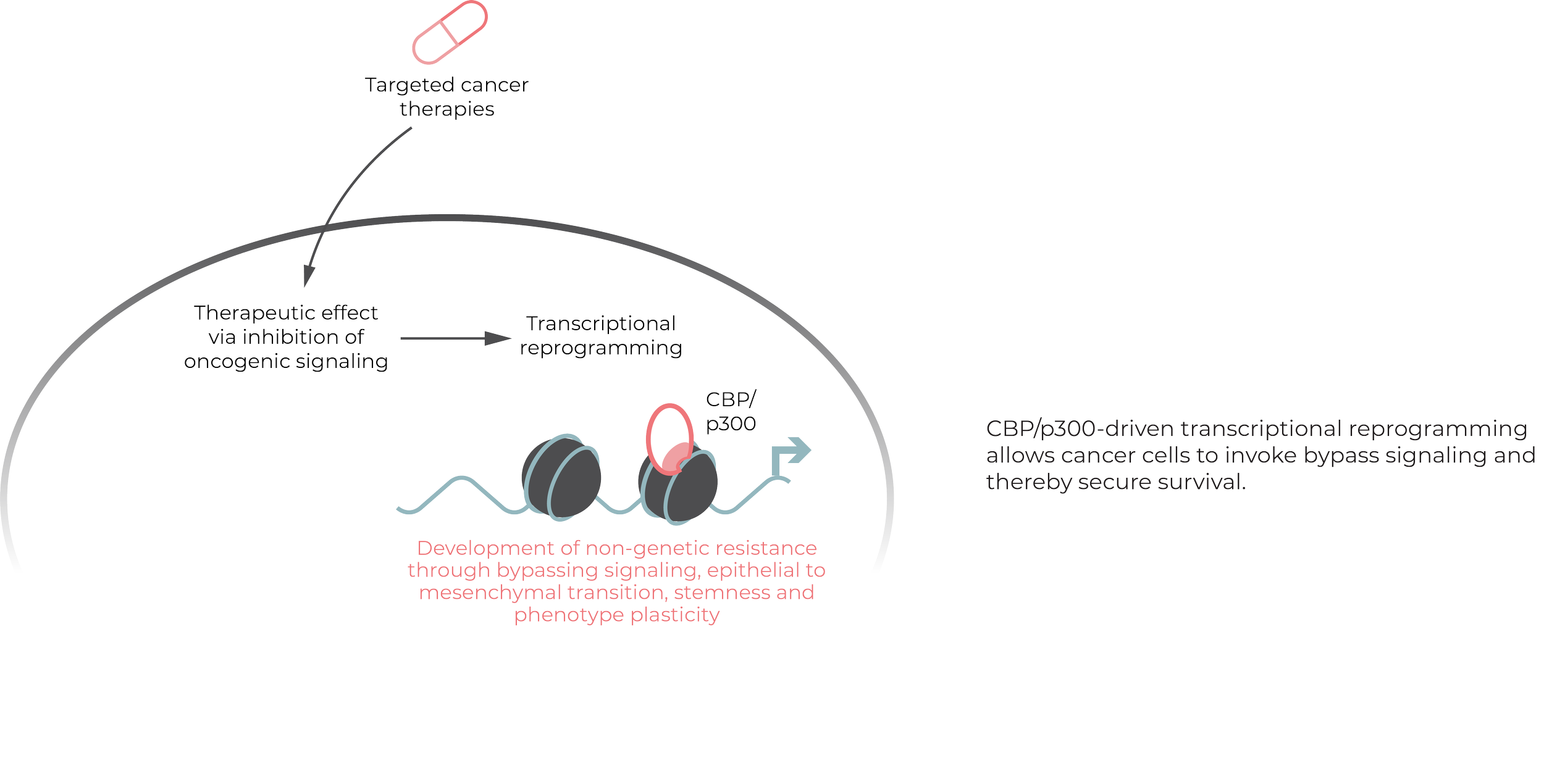
Cancer drug resistance is a major impediment to the long-term survival of patients and is often the primary driver of relapse. While genetic mutations eventually render cancer impervious to treatment, non-genetic resistance mechanisms can be overturned. By targeting early transcriptional reprogramming, cancer's first defense to targeted treatments, our goal is to prevent therapy evasion before it occurs.
Our unique approach to therapeutic target discovery
Aiming to prevent early cancer resistance, we screened for small molecules capable of downregulating non-genetic cancer cell resistance phenotypes and through this approach identified the target CBP/p300 as a novel resistance modulator.
CBP and p300 are highly similar transcriptional coactivators involved in regulation of gene expression. CBP/p300 is a clinically validated target in multiple myeloma, however, it has not been previously implicated in non-genetic cancer drug resistance.
Through our phenotypic screening approach we discovered that CBP/p300 governs critical transcriptional resistance pathways. Our unique approach to target identification and medicinal chemistry, allowed us to developed a compound able to specifically block this critical mechanism upstream of transcriptional escape programs without interfering with cancer-unrelated pathways.
Our lead candidate TT125-802
Our lead candidate TT125-802 is an orally available, selective small molecule inhibitor that targets the bromodomain of the transcriptional co-activators CBP/p300. Preclinical potency, selectivity, and safety data demonstrate that TT125-802 has a highly differentiated profile, providing a new clinical opportunity for an established target.
Mode of Action
Cancer cells leverage transcriptional reprogramming to evade targeted therapy
TT125-802 blocks transcriptional reprogramming and stops early cancer drug resistance
Our clinical development strategy
TT125-802 has the potential to significantly improve targeted treatment durability and bring lasting benefit to patients
We have demonstrated that combining TT125-802 with targeted therapies such as KRAS, EGFR and AR inhibitors promotes deeper and more durable responses in pre-clinical models of non-small cell lung cancer, colorectal cancer, and prostate cancer. We are advancing TT125-802 through a Phase 1 dose-escalation study as a monotherapy in solid tumor patients.